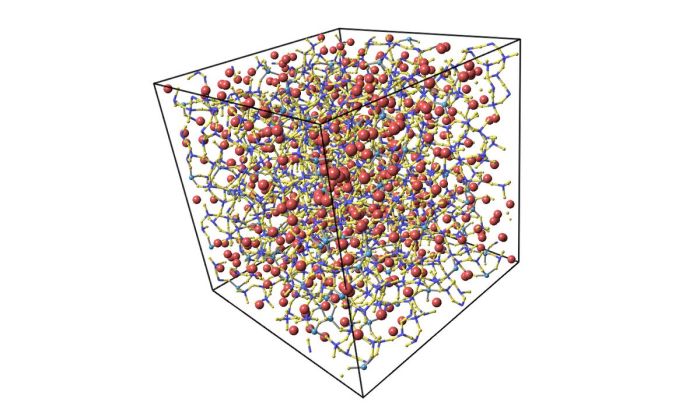
The atomic arrangement of a glass pitcher holds the key to understanding its unique properties and behavior. Unlike crystalline materials with their ordered atomic structure, glass exhibits an amorphous structure, characterized by the random arrangement of atoms. This seemingly chaotic arrangement imparts distinctive characteristics to glass, making it an indispensable material in various applications.
Delving into the atomic bonding within glass reveals the interplay of covalent and ionic bonds. These bonds determine the strength and stability of the glass structure, influencing its resistance to mechanical stress and chemical degradation. Furthermore, the molecular arrangement of glass involves the formation of tetrahedral units, interconnected in a complex network.
The connectivity and distribution of these units shape the overall structure and properties of glass.
Glass Properties: Atomic Arrangement Of A Glass Pitcher
Glass is a unique material with a wide range of properties that make it useful for a variety of applications. These properties include transparency, strength, durability, and chemical resistance. The atomic arrangement of glass plays a crucial role in determining these properties.
In crystalline materials, atoms are arranged in a regular, repeating pattern. In contrast, glass has an amorphous structure, meaning that its atoms are arranged in a random, disordered manner. This random arrangement of atoms gives glass its unique properties.
Amorphous Structure
The amorphous structure of glass is responsible for its transparency. In crystalline materials, light is scattered by the regular arrangement of atoms. In glass, the random arrangement of atoms prevents light from being scattered, allowing it to pass through the material without being absorbed or deflected.
The amorphous structure of glass also makes it strong and durable. In crystalline materials, cracks can propagate easily along the regular arrangement of atoms. In glass, the random arrangement of atoms makes it difficult for cracks to propagate, making it more resistant to breakage.
Atomic Bonding, Atomic arrangement of a glass pitcher
The atomic bonding in glass also contributes to its properties. Glass is formed by the cooling of molten silicate materials. As the molten material cools, the atoms form covalent bonds with each other. Covalent bonds are strong and stable, which contributes to the strength and durability of glass.
In addition to covalent bonds, glass also contains ionic bonds. Ionic bonds are formed between atoms that have lost or gained electrons. Ionic bonds are weaker than covalent bonds, but they contribute to the chemical resistance of glass.
Molecular Arrangement
The molecular arrangement of glass also affects its properties. Glass is composed of tetrahedral units, which are four atoms arranged in a pyramid shape. These tetrahedral units are linked together by covalent bonds to form a continuous network.
The connectivity and distribution of these tetrahedral units affect the overall structure and properties of glass. For example, glasses with a high degree of connectivity are more resistant to crystallization.
Crystallization
Glass can be crystallized by heating it to a high temperature and then cooling it slowly. Crystallization occurs when the atoms in the glass rearrange themselves into a regular, repeating pattern. The rate of crystallization is influenced by the composition of the glass and the cooling rate.
Controlled crystallization can be used to create different types of glass with specific properties. For example, crystallized glass is stronger and more resistant to heat than amorphous glass.
Key Questions Answered
What is the difference between crystalline and amorphous materials?
Crystalline materials possess a highly ordered atomic arrangement, forming a regular lattice structure. In contrast, amorphous materials, like glass, exhibit a random and disordered atomic arrangement.
How does the atomic arrangement affect the properties of glass?
The amorphous structure of glass contributes to its unique properties, such as transparency, low thermal conductivity, and high chemical resistance. The random arrangement of atoms prevents the formation of long-range order, resulting in these distinctive characteristics.
Can the atomic arrangement of glass be controlled?
Yes, controlled crystallization techniques can be employed to manipulate the atomic arrangement of glass, creating different types of glass with specific properties. By controlling the cooling rate and composition, it is possible to induce crystallization, resulting in glass-ceramics with tailored properties.